Fox with bacterial meningitis

Vulpes vulpes
(Photo1, J. Nijendijk, Saxifraga)
In mid-August, an adult male fox (Vulpes vulpes) found dead in woodlands near the Dutch town of Nijmegen was submitted to the DWHC for post-mortem investigation.
Macroscopic findings
Investigation was impaired by the advanced state of decomposition of the carcase, however, it was evident that the animal had a poor nutritional condition (few fat reserves and low muscle mass). Small wounds were found in the skin and one of the external ear canals was filled with a large amount of black crumbly material; the corresponding middle ear and the tympanic bulla contained a small amount of clear fluid (0.5ml). All of the upper and many of the lower incisor teeth were missing. Both spleen and liver were enlarged and pallor was noted in the heart and kidneys in which small red spots were seen scattered throughout the cortex. The membranes covering the brain (meninges) were covered with yellowy, turbid material (mucopurulent exudate) and the cerebrospinal fluid (CSF), that bathes the brain and spinal cord, was cloudy (Photo 2).
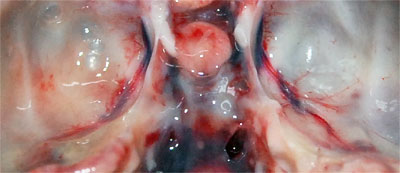 |
Photo 2: The bottom of the inside of the cranium with mucopurulent material partially obscuring the blood vessel on the left. |
Microscopic findings
The meninges were thickened by the presence of a layer of fibrin (protein derived from the blood) mixed with inflammatory cells consisting of granulocytes, lymphocytes, plasma cells and activated histiocytes. In addition, mild mineralisation was seen in the lungs.
Laboratory tests
Bacterial culture: Staphylococcus pseudintermedius and Proteus sp. were cultured from a dry swab of the meninges.
Antibiotic sensitivity testing:. Further testing showed that this strain of S. pseudintermedius was sensitive to a range of antibiotics: amoxyxilline + clavulaanzuur, ampicilline, cefalexine, enrofloxacine, lincomycine, tretracycline en trimethoprim-sulfamethoxazol. These findings show that this strain did not appear to have multi-antibiotic resistance.
Virus testing: As part of routine screening, a sample of brain tissue was tested for rabies virus (PCR) at the Central Veterinary Institute at Wageningen UR (CVI). This animal was negative.
Fecal analysis: Feces contained round worm (nematodes) eggs including Capillaria sp. and Uncinaria sp..
Conclusion
The most likely cause of death of this fox was the severe, subacute, diffuse, mucopurulent meningitis associated with Staphylococcus pseudintermedius and Proteus sp. infections. Given the abnormalities in the ear canal it is believed that the infection spread from the affected ear to the meninges. The poor nutritional status may have been caused by:
- unusual hunting or eating behaviour resulting from the meningitis,
- impaired mastication due to the missing dental elements, and/or
- the presence of gastrointestinal parasites.
Furthermore, the possibility of there being other pathology in the gastrointestinal tract that could have contributed to the poor nutritional status cannot be ruled out due to the degree of decomposition which masks subtle changes.
Bacterial meningitis
Etiology
Meningitis can be caused by bacteria (as in this case), viruses, fungi, protozoa and other parasites.
Epidemiology
S. pseudintermedius and Proteus sp. are ubiquitous and are considered to be opportunistic pathogens (i.e. infection does not typically result in disease). However, in some situations, Staphylococcus sp. can cause serious disease in a range of species. S. pseudintermedius was only recently differentiated from S. aureus and other sorts of staphylococci [4]. It is a normal commensal of the skin and mucosa of cats and dogs and can be associated with disease in the skin, body cavities and other tissues [10]. Proteus sp. are frequently found in the environment (soils, water and feces) and are part of the intestinal flora of normal animals [1, 7]. In some circumstances they are associated with urinary tract infections, bacteremia [1, 7], wound infections and meningitis [3, 6].
Pathogenesis and clinical signs
Bacterial meningitis can result from the spread of infection from a wound, nasal sinusses or the ears. Typical signs include:
- fever,
- hyperesthesia (an increased sensitivity to stimuli),
- stiff neck,
- spasm of the paraspinal musculature,
- photophobia (intolerance to light sources).
Depending on the speed with which infection takes hold and the location of the lesions, behavioural signs may also be seen [2]:
- depression, or agitation
- progressive paresis,
- epileptic fits,
- coma.
Diagnosis
In dead wild animals diagnosis is based on post-mortem findings such as those described here, supported by bacterial culture of the CSF. In the case of Staphylococcal infection, genotyping is necessary to distinguish between S. pseudintermedius and S. aureus [9, 10].
Prevention and control
Meningitis cases caused by opportunisitic bacteria tend to be incidental findings encountered during post-mortem and, therefore, whilst severe for the individual, do not typically impact population health. Opportunistic bacteria can also infect humans and pets and treatment can sometimes be complicated by the presence of antibiotic resistant strains of these bacteria [5, 8, 10]; Staphylococcus sp. are considered a threat to public health [10]. Multi-antibiotic resistance in Proteus sp. is relatively rare in the Netherlands compared to other parts of the world [5].
References
- 2004. Infections of the central nervous system. 3rd ed. Scheld W.M. Whitley RJ. and Marra C.M. eds. Lippincott Williams & Wilkins. 939 pp.
- 2005. The Merck Veterinary Manual. 9th ed. Kahn CM. & Line S. eds. Merck & Co., Inc. 2712 pp.
- Chuang Y. Chang W. Lu C. and Huang C. 2000. Mixed Infection in Adult Bacterial Meningitis. Infection, 28, 1: 8-12.
- Haesebrouck F. Devriese L.A. Vancanneyt M. Baele M. Vaneechoutte M. De Graef E. Snauwaert C. Cleenwerck I. Dawyndt P. Swings J. and Decostere A. 2005. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int. J. Syst. Evol. Microbiol. 55, 1569–1573.
- Hoogkamp-Korstanje J.A.A. Roelofs-Willemse J. and The Susceptibility Surveillance Study Group. 2003. Antimicrobial resistance in Gram-negative bacteria from Intensive Care Units and Urology Services. A nationwide study in The Netherlands 1995-2000. International journal of antimicrobial agents, 21, 6: 547-556.
- Kassim Z. Aziz A.A. and Haque Q.M. 2003. Isolation of Proteus mirabilis from severe neonatal sepsis and central nervous system infection with extensive pneumocephalus. European journal of pediatrics, 162, 9: 644-645.
- Kim B.N. Kim, N.J. Kim, M.N. Kim Y.S. Woo J.H. and Ryu J. 2003. Bacteraemia due to tribe Proteeae: a review of 132 cases during a decade (1991-2000). Scandinavian Journal of Infectious Diseases, 35, 2: 98-103. Available on: http://informahealthcare.com/doi/pdf/10.1080/0036554021000027015
- Steen S. 2011. Meticillin-resistant strains of Staphylococcus pseudintermedius in companion animals. Veterinary record, 169, 2: 53-54.
- Van Hoovels L. Vankeerberghen A. Boel A. Van Vaerenbergh K. and De Beenhouwer H. 2006. First Case of Staphylococcus pseudintermedius Infection in a Human. Journal of clinical microbiology, 44, 12: 4609–4612.
- Weese J.S. and van Duijkeren E. 2010. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Veterinary microbiology, 140, 3-4: 418-429.



